Three new vaccines for bluetongue, which have been approved for use in the UK, will be initially targeted at high-risk areas in southeast England.
“We want to make sure that those at greatest risk get first dibs at that vaccine,” Gordon Hickman from Defra said during an online briefing.
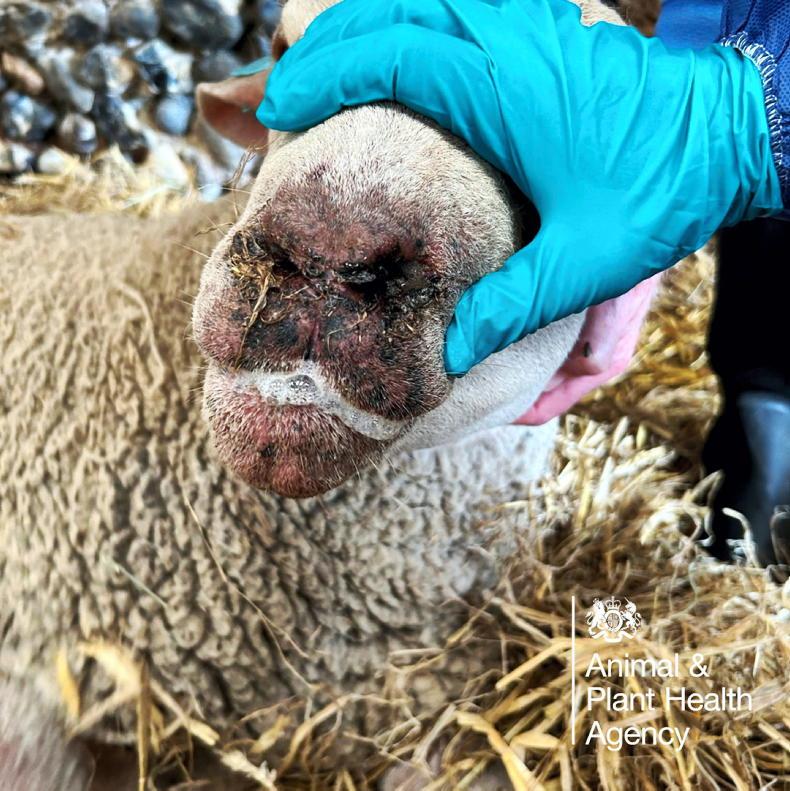
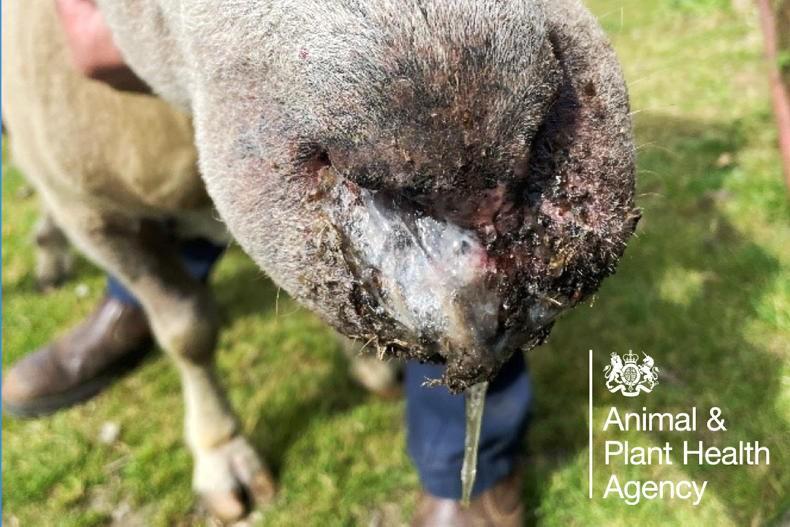
More outbreaks of the disease have occurred in England in recent days, with the BTV-3 strain of the virus confirmed on 38 farms by Wednesday afternoon.
Hickman said rollout of the new vaccines will depend on both supply and demand, so a phased approach is being taken initially.
“If the take up is low and we have loads of supply, then we will open up that general licence probably along the south coast next, then make it available to the rest of England,” he said.
The Defra official pointed out the vaccines suppress bluetongue in livestock and are not effective at preventing cattle and sheep picking up infections.
“There is good evidence that they reduce clinical signs and there is evidence that mortality rates are reduced,” Hickman said.
“Even if you vaccinate livestock, we will still treat them as unvaccinated for the purposes of disease control, so trade restrictions and movement controls will still apply,” he added.
Devolved
Even though last week’s approval of the vaccines is UK-wide, the decision to use the vaccines under licence rests with different devolved administrations.
A spokesperson for DAERA confirmed that there are no immediate plans to roll out BTV-3 vaccines in NI at present, although the matter is being kept under review.
“DAERA officials will continue to monitor the development of vaccine use in England, working closely with stakeholders across NI and colleagues in the Republic of Ireland to determine next steps,” the spokesperson said.







SHARING OPTIONS