Aphid control is reliant on several overlapping approaches, such as planting date and variety, the correct timing of insecticides or managing ‘green-bridge’ transmission.
Additionally, other factors such as climate change, partially insecticide-resistant aphids and the ever-decreasing availability of active ingredients and modes of action will impede successful aphid management in the future.
Teagasc has recently established an aphid suction tower network and new analytical laboratory methods to assist in the monitoring of migrating aphids and crop diseases caused by plant viruses.
This investment will deliver greater insight into the potential for disease prevalence, which will assist and inform already established integrated pest management (IPM) approaches.
BYDV and insecticide resistance
The grain aphid Sitobion avenae and the bird cherry-oat aphid Rhopalosiphum padi are the main vectors of the barley yellow dwarf virus (BYDV).
To help minimise the damage caused by the disease, farmers have to rely on insecticides, in combination with drilling date and ‘green-bridge’ management, to control the aphids which transmit and spread the virus in the crop.
Aphid control remains essential as there is no direct control for the virus in the plant.
Currently, the options for aphid control with insecticides are limited to pyrethroid sprays, as seed treatment with neonicotinoids were restricted in the EU since 2018.
In 2013, partially pyrethroid-resistant grain aphids were detected after spray failures in Ireland and these have been detected throughout the main grain-growing counties since.
This partial insecticide resistance is caused by a mutation in parts of the aphid’s nervous system (knockdown resistance, kdr), with the consequence that pyrethroids cannot paralyse the insect efficiently. This results in a higher proportion of the surviving aphids carrying the resistance following spray application.
As well as the kdr-mutation, preliminary data from Teagasc laboratory studies has shown that some populations of the partially resistant aphid clones recovered from tillage crops, have also developed detoxifying enzymes which allow them to degrade the insecticide and therefore survive higher insecticide rates.
This evidence for an enhanced metabolic resistance may cause additional problems in the future.
Impact of migrating aphids is unknown
The current approach to aphid management with pyrethroids depends on observable aphid levels in field in combination with weather conditions.
This is because infected plants only display symptoms after infection when damage to the plant has already occurred.
There is currently a knowledge gap as to when critical levels of BYDV (virus threshold) are reached in a field and the relevant impact of migrating aphids in the spread of BYDV. In terms of BYDV infection in newly emerging crops, little is certain about the epidemiology of the virus in Ireland.
However, migrating aphids and/or aphids carried over from previous crops are thought to be the reinfection routes.
It is necessary to differentiate between primary infection through virus-carrying flying aphids (these introduce the virus into an uninfected field) and secondary infection (aphids spread the already present virus from infected plants within the field) (see Table 1).
Also, the impact of in-field movement (aphids fly or hop very short distances within a field or from green bridges) and the impact of landscape-level migration (aphids take off for a longer flight to explore new food resources or fly to their winter host) needs to be further investigated.
Currently, the importance of these aphid movement events and the interaction with virus infection routes at local and landscape levels is unknown.
This highlights the benefit of the suction tower monitoring network, as it allows us to catch migrating aphids and analyse them to understand if they are carrying the virus, in order to unravel this puzzle.
How does a suction tower
network work?
The suction tower network is based on the design from the Rothamsted Research Aphid Monitoring network, which is the longest-running insect experiment in the world. To help understand the impact of aphid movement and migration on BYDV spread, a network of three suction towers is being established in Ireland.
Units in Carlow and Cork are already operational and an additional tower is to be installed in north Dublin in 2021 (Figures 1 and 2).

Figure 1
Work from Rothamsted in the 1970s indicated that the aphid catch in each suction tower is reflective of the aphid catches from an approximately 80km diameter around the tower.
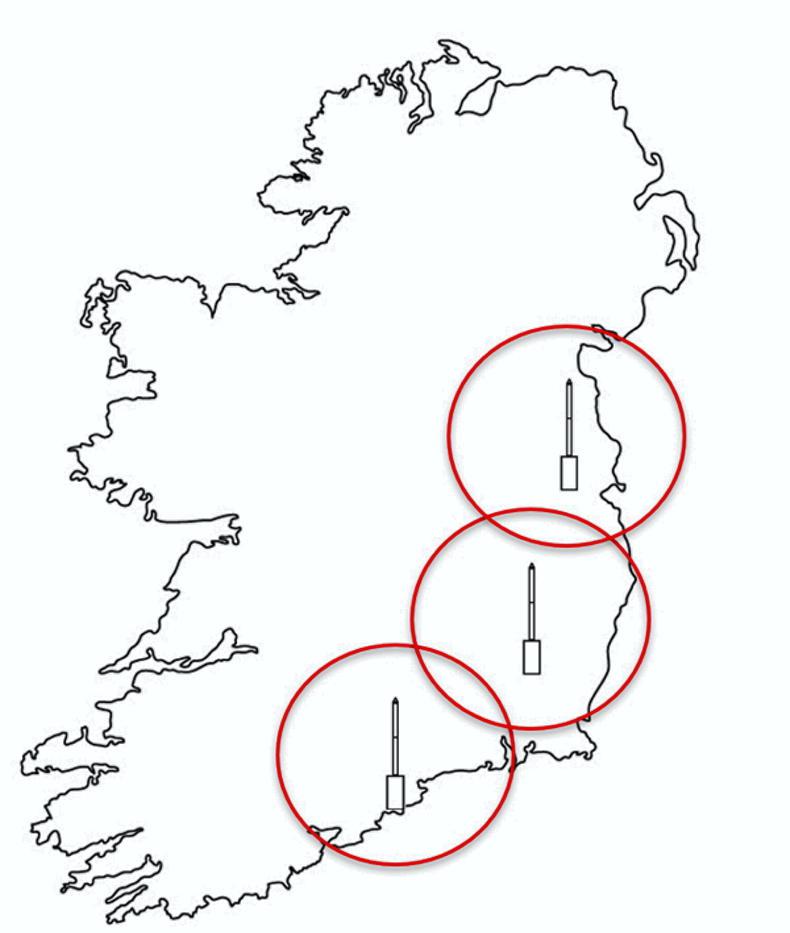
Figure 2
Therefore, the network will allow us to make observations which cover much of the east, southeast and south of Ireland.
Each 12.2m high suction tower consists of a long pipe through which aphids and other insects get sucked down into collection bottles (Figure 3).

Figure 3
These rotate on a carousel to allow continuous sampling on a daily basis and collected aphids are separated from other insects and stored for laboratory analysis.
Once fully operational, the combination of the tower network and the increased molecular diagnostic capacity will allow us to:
Understand the levels of migrating aphids carrying viruses, identify the time period when virus carrying aphids may be prominent and associate the relationship between these levels and BYDV occurrence in fields.Link aphid migration to weather factors such as temperature, rainfall, and wind speed and to examine the potential to predict aphid migration based on weather forecast and long-range forecasts in the future.Determine the levels of insecticide resistance in populations of migrating aphids, which will inform disease management strategies based on real-time data.Aphid migration in 2020
Last year (2020) was the first year the first suction tower ran continuously in Oak Park and the numbers of migrating (flying) aphids were found to be affected by different weather conditions (see Table 2).
The bird cherry-oat aphid was the most abundant species recovered and was present until the end of November. The grain aphid occurred in lower numbers and only until the end of August.
Aphids generally start to fly and migrate at temperatures around 15°C and the observations showed that they start to migrate in high numbers when the weekly average temperature is above 15°C. Also, as expected, strong winds and rain had a negative effect on aphid flight, as aphids cannot take off in unfavourable conditions.
In 2017 and 2018, between 20% and 25% of grain aphids recovered from tillage fields around the country tested positive for the gene mutation conferring partial insecticide resistance (kdr).
In 2020, grain aphids recovered from the Oak Park suction tower showed a similar incidence rate of 22%. This indicated that partially resistant aphids are still present in the population and capable of migrating large distances.
It should also be noted that 78% of the grain aphid population did not carry the resistant gene and should therefore be fully susceptible to a pyrethroid insecticide.
Given the withdrawal of neonicotinoid insecticides and our large reliance on pyrethroids, which the kdr mutation is specific to, monitoring the ratio of partially resistant to susceptible grain aphid clones into the future will be of particular interest. Molecular analysis also indicated that these aphids are only partially resistant and there is no evidence yet of a fully resistant clone.
In 2021, the suction tower network will be extended further, which will allow for increased regional monitoring.
New detection technologies
The suction tower network will be a powerful tool, not only to study migrating aphids and their resistance status, but also to collect aphids in a continual and consistent manner. This will then allow further investigations into their role as plant virus vectors and their involvement in the epidemiology of plant viruses in general.
Teagasc recently has acquired a new digital droplet PCR (ddPCR) system. This allows for the exact quantification of virus within a sample (eg aphids or leaves), where previous technology only allowed us to determine if the virus was present or absent.
This new ddPCR technology can be used to detect low amounts of BYDV in aphids and in plant leaves (see Figure 4).

Figure 4
Sampling of barley leaves and aphids will begin this spring and this information will help us to better understand the epidemiology of BYDV, especially in periods when virus levels are low and when there are no symptomatic plants.
Potential impact for IPM
Our current IPM strategy is based on multiple approaches, which include appropriate insecticide applications. The overall aim of this investment is to further support these IPM approaches by providing growers with more timely information about BYDV levels in fields and aphid migration.
By combining in-field and landscape-scale aphid monitoring with new technologies to detect the virus in plants and aphids, we can gather the information required to predict BYDV occurrence and the occurrence of insecticide-resistant aphid populations.
Our current IPM strategy is based on multiple approaches, which include appropriate insecticide applications
This will expand our ability to successfully advise on IPM approaches and insecticide spray decisions for improved management of aphids and BYDV in the future.
The combination of the suction tower network and the ddPCR also have potential to monitor and analyse other aphids and viruses, as well as grain aphids and BYDV.
There are many other aphids and plant viruses which affect agriculture and horticulture, such as the Peach Potato Aphid Myzus persicae, a main vector of crop damaging viruses such as potyviruses in potatoes and turnip yellow virus in brassicas, to name but a few.
The suction tower network infrastructure will therefore benefit the industry as a whole.
The information it will help generate on the impact of migrating aphid species on crop yields and quality will help us to develop the knowledge necessary for the development and deployment of future aphid IPM approaches.
Aphid control is reliant on several overlapping approaches, such as planting date and variety, the correct timing of insecticides or managing ‘green-bridge’ transmission.
Additionally, other factors such as climate change, partially insecticide-resistant aphids and the ever-decreasing availability of active ingredients and modes of action will impede successful aphid management in the future.
Teagasc has recently established an aphid suction tower network and new analytical laboratory methods to assist in the monitoring of migrating aphids and crop diseases caused by plant viruses.
This investment will deliver greater insight into the potential for disease prevalence, which will assist and inform already established integrated pest management (IPM) approaches.
BYDV and insecticide resistance
The grain aphid Sitobion avenae and the bird cherry-oat aphid Rhopalosiphum padi are the main vectors of the barley yellow dwarf virus (BYDV).
To help minimise the damage caused by the disease, farmers have to rely on insecticides, in combination with drilling date and ‘green-bridge’ management, to control the aphids which transmit and spread the virus in the crop.
Aphid control remains essential as there is no direct control for the virus in the plant.
Currently, the options for aphid control with insecticides are limited to pyrethroid sprays, as seed treatment with neonicotinoids were restricted in the EU since 2018.
In 2013, partially pyrethroid-resistant grain aphids were detected after spray failures in Ireland and these have been detected throughout the main grain-growing counties since.
This partial insecticide resistance is caused by a mutation in parts of the aphid’s nervous system (knockdown resistance, kdr), with the consequence that pyrethroids cannot paralyse the insect efficiently. This results in a higher proportion of the surviving aphids carrying the resistance following spray application.
As well as the kdr-mutation, preliminary data from Teagasc laboratory studies has shown that some populations of the partially resistant aphid clones recovered from tillage crops, have also developed detoxifying enzymes which allow them to degrade the insecticide and therefore survive higher insecticide rates.
This evidence for an enhanced metabolic resistance may cause additional problems in the future.
Impact of migrating aphids is unknown
The current approach to aphid management with pyrethroids depends on observable aphid levels in field in combination with weather conditions.
This is because infected plants only display symptoms after infection when damage to the plant has already occurred.
There is currently a knowledge gap as to when critical levels of BYDV (virus threshold) are reached in a field and the relevant impact of migrating aphids in the spread of BYDV. In terms of BYDV infection in newly emerging crops, little is certain about the epidemiology of the virus in Ireland.
However, migrating aphids and/or aphids carried over from previous crops are thought to be the reinfection routes.
It is necessary to differentiate between primary infection through virus-carrying flying aphids (these introduce the virus into an uninfected field) and secondary infection (aphids spread the already present virus from infected plants within the field) (see Table 1).
Also, the impact of in-field movement (aphids fly or hop very short distances within a field or from green bridges) and the impact of landscape-level migration (aphids take off for a longer flight to explore new food resources or fly to their winter host) needs to be further investigated.
Currently, the importance of these aphid movement events and the interaction with virus infection routes at local and landscape levels is unknown.
This highlights the benefit of the suction tower monitoring network, as it allows us to catch migrating aphids and analyse them to understand if they are carrying the virus, in order to unravel this puzzle.
How does a suction tower
network work?
The suction tower network is based on the design from the Rothamsted Research Aphid Monitoring network, which is the longest-running insect experiment in the world. To help understand the impact of aphid movement and migration on BYDV spread, a network of three suction towers is being established in Ireland.
Units in Carlow and Cork are already operational and an additional tower is to be installed in north Dublin in 2021 (Figures 1 and 2).

Figure 1
Work from Rothamsted in the 1970s indicated that the aphid catch in each suction tower is reflective of the aphid catches from an approximately 80km diameter around the tower.

Figure 2
Therefore, the network will allow us to make observations which cover much of the east, southeast and south of Ireland.
Each 12.2m high suction tower consists of a long pipe through which aphids and other insects get sucked down into collection bottles (Figure 3).

Figure 3
These rotate on a carousel to allow continuous sampling on a daily basis and collected aphids are separated from other insects and stored for laboratory analysis.
Once fully operational, the combination of the tower network and the increased molecular diagnostic capacity will allow us to:
Understand the levels of migrating aphids carrying viruses, identify the time period when virus carrying aphids may be prominent and associate the relationship between these levels and BYDV occurrence in fields.Link aphid migration to weather factors such as temperature, rainfall, and wind speed and to examine the potential to predict aphid migration based on weather forecast and long-range forecasts in the future.Determine the levels of insecticide resistance in populations of migrating aphids, which will inform disease management strategies based on real-time data.Aphid migration in 2020
Last year (2020) was the first year the first suction tower ran continuously in Oak Park and the numbers of migrating (flying) aphids were found to be affected by different weather conditions (see Table 2).
The bird cherry-oat aphid was the most abundant species recovered and was present until the end of November. The grain aphid occurred in lower numbers and only until the end of August.
Aphids generally start to fly and migrate at temperatures around 15°C and the observations showed that they start to migrate in high numbers when the weekly average temperature is above 15°C. Also, as expected, strong winds and rain had a negative effect on aphid flight, as aphids cannot take off in unfavourable conditions.
In 2017 and 2018, between 20% and 25% of grain aphids recovered from tillage fields around the country tested positive for the gene mutation conferring partial insecticide resistance (kdr).
In 2020, grain aphids recovered from the Oak Park suction tower showed a similar incidence rate of 22%. This indicated that partially resistant aphids are still present in the population and capable of migrating large distances.
It should also be noted that 78% of the grain aphid population did not carry the resistant gene and should therefore be fully susceptible to a pyrethroid insecticide.
Given the withdrawal of neonicotinoid insecticides and our large reliance on pyrethroids, which the kdr mutation is specific to, monitoring the ratio of partially resistant to susceptible grain aphid clones into the future will be of particular interest. Molecular analysis also indicated that these aphids are only partially resistant and there is no evidence yet of a fully resistant clone.
In 2021, the suction tower network will be extended further, which will allow for increased regional monitoring.
New detection technologies
The suction tower network will be a powerful tool, not only to study migrating aphids and their resistance status, but also to collect aphids in a continual and consistent manner. This will then allow further investigations into their role as plant virus vectors and their involvement in the epidemiology of plant viruses in general.
Teagasc recently has acquired a new digital droplet PCR (ddPCR) system. This allows for the exact quantification of virus within a sample (eg aphids or leaves), where previous technology only allowed us to determine if the virus was present or absent.
This new ddPCR technology can be used to detect low amounts of BYDV in aphids and in plant leaves (see Figure 4).

Figure 4
Sampling of barley leaves and aphids will begin this spring and this information will help us to better understand the epidemiology of BYDV, especially in periods when virus levels are low and when there are no symptomatic plants.
Potential impact for IPM
Our current IPM strategy is based on multiple approaches, which include appropriate insecticide applications. The overall aim of this investment is to further support these IPM approaches by providing growers with more timely information about BYDV levels in fields and aphid migration.
By combining in-field and landscape-scale aphid monitoring with new technologies to detect the virus in plants and aphids, we can gather the information required to predict BYDV occurrence and the occurrence of insecticide-resistant aphid populations.
Our current IPM strategy is based on multiple approaches, which include appropriate insecticide applications
This will expand our ability to successfully advise on IPM approaches and insecticide spray decisions for improved management of aphids and BYDV in the future.
The combination of the suction tower network and the ddPCR also have potential to monitor and analyse other aphids and viruses, as well as grain aphids and BYDV.
There are many other aphids and plant viruses which affect agriculture and horticulture, such as the Peach Potato Aphid Myzus persicae, a main vector of crop damaging viruses such as potyviruses in potatoes and turnip yellow virus in brassicas, to name but a few.
The suction tower network infrastructure will therefore benefit the industry as a whole.
The information it will help generate on the impact of migrating aphid species on crop yields and quality will help us to develop the knowledge necessary for the development and deployment of future aphid IPM approaches.

















SHARING OPTIONS