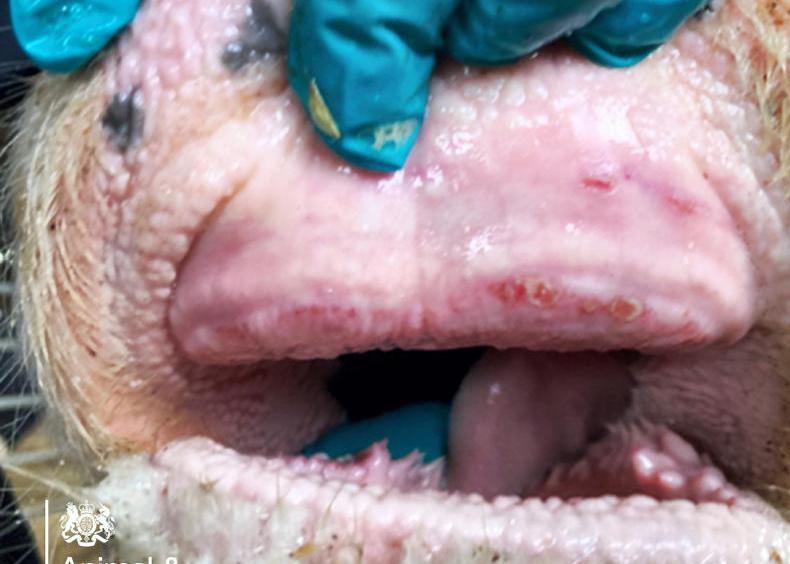
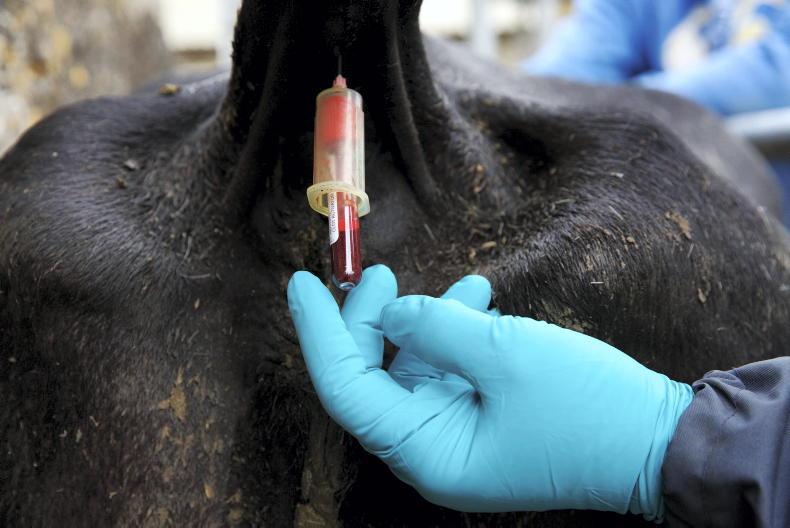
The confirmation of bluetongue virus in two animals and 44 further suspect cases on a farm in Northern Ireland has sent shockwaves through the farming community.
There is an increasing number of questions arising regarding vaccination.
There are three vaccines approved for use in Northern Ireland and Britain – Bluevac-3, Bultavo 3 and Syvazul BTV 3. The three vaccines were approved for use in June 2025 but reports indicate the uptake was low with many veterinary practitioners only moving in recent days to source supplies.
The uptake of the vaccine is also likely to remain variable in the short-term with many farmers waiting to see if further cases are identified in the coming days. If this is the case then it is likely that there will be a push for greater vaccination ahead of the season for increased midge activity.
Veterinary prescription
The vaccine must be prescribed by a veterinary practitioner but can be administered by the keep of animal who will be administered with a licence by the prescribing vet. There are also additional obligations in terms of the range of records that must be maintained for animals which are vaccinated.
DAERA outline that written or digital records must be kept of every animal vaccinated and must be provided to an inspector on request. The record must be retained by the keeper for at least five years and contain the following:
Species vaccinated.Individual livestock identification number (as per livestock identification tag). Date of first vaccine administered.Date of any further doses.Vaccine product name and batch number.Name and job title of the person who administered the vaccine.Dose administered (ml).Route of administration (i.e. intramuscularly or subcutaneously).The stated withdrawal period of the vaccine used.DAERA adds that once the vaccine has been administered, the person to whom this licence is granted must report the following details to their local DAERA Direct Office within five working days:
Specific licence reference number.Individual livestock identification numbers of the animals that the vaccine was administered to.The date the vaccine was administered.A summary overview of the three vaccines as per the summary of product characteristics published by the UK government is outlined below. Further information on the virus and vaccination can be found at https://www.gov.uk/guidance/bluetongue-serotype-3-btv-3-vaccination.
Read more
No plans in NI to cull BTV-3 cattle
‘Unusual' and 'localised' midge activity behind NI bluetongue cases - UFU
The confirmation of bluetongue virus in two animals and 44 further suspect cases on a farm in Northern Ireland has sent shockwaves through the farming community.
There is an increasing number of questions arising regarding vaccination.
There are three vaccines approved for use in Northern Ireland and Britain – Bluevac-3, Bultavo 3 and Syvazul BTV 3. The three vaccines were approved for use in June 2025 but reports indicate the uptake was low with many veterinary practitioners only moving in recent days to source supplies.
The uptake of the vaccine is also likely to remain variable in the short-term with many farmers waiting to see if further cases are identified in the coming days. If this is the case then it is likely that there will be a push for greater vaccination ahead of the season for increased midge activity.
Veterinary prescription
The vaccine must be prescribed by a veterinary practitioner but can be administered by the keep of animal who will be administered with a licence by the prescribing vet. There are also additional obligations in terms of the range of records that must be maintained for animals which are vaccinated.
DAERA outline that written or digital records must be kept of every animal vaccinated and must be provided to an inspector on request. The record must be retained by the keeper for at least five years and contain the following:
Species vaccinated.Individual livestock identification number (as per livestock identification tag). Date of first vaccine administered.Date of any further doses.Vaccine product name and batch number.Name and job title of the person who administered the vaccine.Dose administered (ml).Route of administration (i.e. intramuscularly or subcutaneously).The stated withdrawal period of the vaccine used.DAERA adds that once the vaccine has been administered, the person to whom this licence is granted must report the following details to their local DAERA Direct Office within five working days:
Specific licence reference number.Individual livestock identification numbers of the animals that the vaccine was administered to.The date the vaccine was administered.A summary overview of the three vaccines as per the summary of product characteristics published by the UK government is outlined below. Further information on the virus and vaccination can be found at https://www.gov.uk/guidance/bluetongue-serotype-3-btv-3-vaccination.
Read more
No plans in NI to cull BTV-3 cattle
‘Unusual' and 'localised' midge activity behind NI bluetongue cases - UFU







SHARING OPTIONS